What is glucagon?
Glucagon is a hormone that promotes the breakdown of glycogen to glucose, making it useful in raising blood glucose levels during a hypoglycaemic episode.
Types of glucagon
| Traditional glucagon kits1 |
| FDA Approval 1988 with no age limitations Administration & Details
Recommended Dosage
|
| Nasal glucagon1 |
| FDA Approval 2019 Administration & Details
Recommended Dosage
|
| Liquid-stable glucagon1 |
| FDA Approval 2019 for paediatric and adult patients Administration & Details
Recommended Dosage
|
| Subcutaneous dasiglucagon2 |
| FDA Approval 2021 Administration & Details
Recommended Dosage
|
| Glucagon Type | FDA Approval | Administration & Details | Recommended Dosage |
|---|---|---|---|
| Traditional glucagon kits1 | 1988 with no age limitations |
|
|
| Nasal glucagon1 | 2019 |
|
|
| Liquid-stable glucagon1 | 2019 for paediatric and adult patients |
|
|
| Subcutaneous dasiglucagon2 | 2021 |
|
|
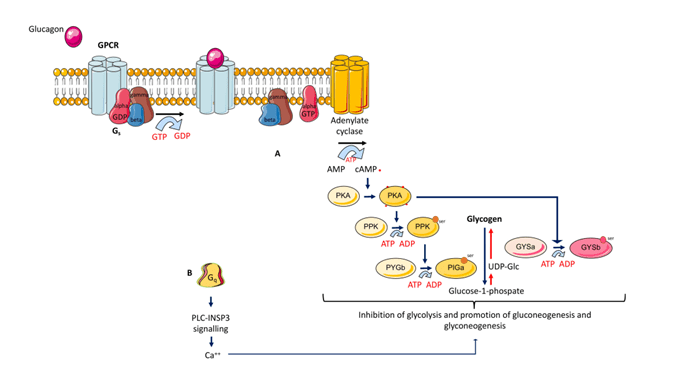
Mechanism of glucagon action, from La Sala & Pontiroli. 2021.2
Glucagon formulations undergoing clinical trials
Biochaperone glucagon3
- Recombinant human glucagon
- Prevents glucagon from degrading by adding compounds that form stable complexes with glucagon
Glucagon limitations
While useful, there are significant limitations to glucagon formulations. Glucagon formulations expire and are complicated to reconstitute. However, they are still used in many countries that don’t have access to nasal or pre-filled stable products.
References
- 1Sherman JJ, Lariccia JL. Glucagon Therapy: A Comparison of Current and Novel TreatmentsDiabetes Spectr. 2020;33:347–351.
- 2La Sala L & Pontiroli AE. New Fast Acting Glucagon for Recovery from Hypoglycemia, a Life-Threatening Situation: Nasal Powder and Injected Stable Solutions. 2021;22:10643.
- 3Beato-Vibora PI & Arroyo-Diez FJ. New uses and formulations of glucagon for hypoglycaemia. Drugs Context. 2019;8:212599.



